
Professor Emeritus
siwoo@kaist.ac.kr
+82-42-350-3918
- 1983 : Univ. of Wisconsin-Madison (Ph.D. in Chem. Eng.)
- 1975 : KAIST (M.S. in Chem. Eng.)
- 1973 : Seoul National Univ. (B.S. in Chem. Eng.)
- 2001 ~ Present : Director, Center for Ultramicrochemical Process Systems
- 1985 ~ Present : Professor, KAIST
- 1990 ~ 1991 : Humboldt Research Professor, Max Planck Inst (Fritz-Haber-Inst.)
- 1989 ~ 1989 : Visiting Professor. Tokyo Institute of Technology
- 1983 ~ 1985 : 3M Company Postdoctoral Fellow, Univ. of Toronto
- 1975 ~ 1978 : Researcher, Polymer Lab. KIST
- Yeosan Catalysis Award, Korean Institute of Chemical Engineers (2006)
- Presidential Korean Engineering Prize, Ministry of Science & Technology (2005)
- National Medal of Science and Technology, Jin-Bo-Jang (2001)
- Academic Award, Korean Institute of Chemical Engineers (1997)
- Excellent Paper Award, The Korean Federation of Science and Technology Societies (1994)
- Combinatorial approach for ferroelectric material libraries prepared by liquid source misted chemical deposition method, P. Natl. Acad. Sci. USA, 104, 1134-1139 (2007).
- Recent Advances in Catalytic DeNOx Science and Technology, Catal. Rev., 48, 43-89 (2006).
- Platinum nanoclusters studded in microporous nano wall of ordered mesoporous carbon, Adv. Mater., 17, 446-451 (2005).
- Glycerol as a bio-derived sustainable fuel for solid oxide fuel cell with internal reforming, ChemSUSChem, 2(11), 1028-1031 (2009).
- Composition optimization of PtRuM/C (M = Fe and Mo) catalysts for methanol electro-oxidation via combinatorial method, Appl. Catal. B : environ., 91, 428-433 (2009)
Combinatorial method, Fuel Cell, Solar Cell, Catalysis, Biomanss Conversion, Olefin polymerization, Transparent Electrode, Semiconductor & Energy Materials
Research interests
- High Throughput Screening augmented by AI
- Ziegler-Natta and Metallocene Olefin Polymerization Catalyst
- Petrochemical and Environmental Catalysis
- CO2 Reduction and Water Splitting
- Fuel Cell
- Nano Materials
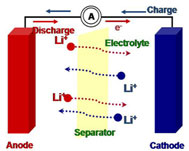
1) Functional additives to modify electrode/electrolyte interface
SEI(solid-electrolyte interface) formed on both electrodes determines cell performance, so controlling electrode/electrolyte interface is necessary. Electrolyte additive can be one of the most effective and simple methods to enhance the cycle performance. Various kinds of electrolytes and their effects on newly developed electrodes are under intensive investigation.
2) Stable lithium electrode by control of lithium metal surface
Lithium metal has a high energy density due to a high specific capacity(3860mAh/g) and low redox potential (-3.04V vs. NHE). However, high reactivity and dendrite growth of lithium on the surface caused unexpected electrochemical behavior such as capacity loss, low cycling efficiency, poor cycle performance, and safety problems. To overcome this problem, the control of lithium metal electrode/ electrolyte interface by using protection layer or functional additive is required.
3) Polymer electrolyte and separator
Polymer electrolyte has higher safety than liquid electrolyte. However, relatively low ionic conductivity is a barrier to the commercialization of polymer electrolyte. To overcome this problem, increase in the ionic conductivity of polymer electrolyte without loss of mechanical strength is required. Separator plays an important role in lithium battery system because it prevent the direct contact of cathode and anode. However, conventional separator has poor compatibility with liquid electrolyte and it’s cost is expensive. In this point, alternative separator materials are required. Additionally, conventional polyolefin separators show large thermal shrinkage at high temperature, which can lead to an internal short in battery at abused conditions. Researches are also focused on improving the dimensional stability of separator at high temperature.
Polymer Electrolyte Fuel Cell (PEFC)
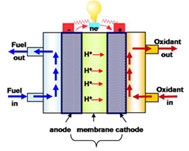
1) Morphology control of catalyst layer based on new electrode binder
The performance of electrode is determined by the reactant transfer properties and the kinetics of the electrochemical reaction. As a key component of electrode, the introduction of new electrode binder causes change of the pore structure in the catalyst layer, it is essential to control morphology of catalyst layer to obtain high performance electrode.
2) Polymer electrolyte membrane with improved durability
Hydrocarbon polymer membranes show certain advantages such as low-cost and methanol permeability, however, they also have barriers for practical application: higher water uptake, poor dimensional and chemical/mechanical stability in wet state. Blend membrane with hydrophobic materials (i.e. PVdF) is very effective in achieving a good dimensional stability and improved long-term stability. Composite membrane using porous matrix is also designed to improve membrane durability. It is composed of a porous matrix and a polymer that fills the pores of the porous matrix. The porous matrix exhibits high dimensional stability due to a mechanical suppression of polymer expansion by hydration.
3) Interface control for long-term stability
The degradation in fuel cell performance is caused by many important factors including losses in catalytic activity, proton conductivity, reactant transfer, and so on. One of the most critical reasons why these phenomena occur is the interfacial failure (i.e. delamination) between the membrane and the electrode. In general, the membrane and the electrode are assembled by physical factors such as heat and pressure. In our lab, as one approach to develop MEA with good interfacial adhesion, the research on the chemically interlocked MEA are ongoing.
Photoelectrochemical Solar Cells
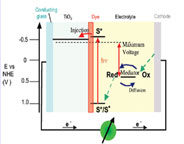
1) Dye-sensitized solar cells (DSSC)
Development of polymer electrolyte
- Long term stability
- High ionic conductivity via doping ionic liquids
- Effect of ionic liquids upon cell performance
- Good interfacial properties
- Semiconductor doped polymer electrolyte
- Alternatives of iodine electrolyte
- Prevention of metal corrosion
- Control of Voc
Synthesis of Quantum dots
- Modifying synthesis route for better assembling
- Surface modification of quantum dots for performance enhancement
Development of new redox couple
- Stability of redox couple between sensitizer and electrolyte
- Improvement of interfacial properties
1) Directional Photofluidization Lithography
“Smaller” brings new capability. Recently, we proposed the directional photofluidization lithography (DPL) as a novel nanolithographic technique that could revolutionize nano-scale photonics, electronics, and energy devices. The key idea of DPL is the use of photo-reconfigurable polymer arrays to be molded in metallic nanostructures instead of conventional colloids or crosslinked polymer arrays. The photo-reconfiguration of polymers by directional photofluidization allows unprecedented control over the sizes and shapes of nanostructures. Besides the capability for precise control of structural features, DPL ensures scalable, parallel, and cost-effective processing, highly compatible with high-throughput fabrication. Therefore, DPL can expand not only the potential for specific metallic nanostructure applications, but also large-scale innovative nanolithography.
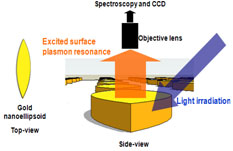
2) Plasmonics
Plasmonics is the study of collective oscillation of electromagnetic wave at the surface of metallic nanostructures. Highly localized electromagnetic waves provide an efficient way for the confinement of light at the level exceeding the diffraction limit. Furthermore, plasmon is very sensitive to the change of refractive index of enviroment. Therefore, if we implement the metallic nanostructures into sensor, we can imagine an ultrasensitive molecolar sensors.
3) Molecular Movement by Optical Field
The ability to control the movement of atoms and nanoscale objectives by means of optical field can open new path towards understanding of matter and the fabrication of photonic devices. In line with this, photochromic materials are one of the most promising materials, since optical pulse-driven changes in the molecolar shapes and dipolemoment can provide efficient ways for the manipolation of atoms and nanoscale objectives. Based on these characteristics of photochromic materials, I focused on understanding of photoinduced motion of photochromic materials including alignment, aggregation, and bulk mass transfer and utilizing such motion in the development of novel superlattice structures that can be used in the areas of nano-optics, chemical/biological sensors, and energy devices.
4) Photoreactive Organic Materials for Holographic Data Storage and Imaging Process
Holography speaks volumes. Recently, holographic imaging has attracted much interest for their wide applications including three-dimensional (3D) visualization, high-density data storage, and nondestructive testing. Among holographic materials, photopolymers have advantages over photorefractive and photoaddressable materials on account of the ability to satisfy both long-term stability of recorded images and high diffraction efficiency. Inspite of their advantages, a volume shrinkage occuring during photopolymerization remains as a major obstacle to the practical application of photopolymers. To address this problem, I aim to develop photopolymers with enhanced dimensional stability as well as high diffraction efficiency.
5) Control of Liquid Crystals Orientation by Molecolar Engineering
Liquid crystals orientation is of practical importance for various optoelectronic devices including displays, sensors, and photonic resonators. In line with this, I try to align the liquid crystals by using Molecular Movement and anisotropic polymeric chain line-up.

