Tuning selectivity of electrochemical reactions by atoomically dispersed platinum catalyst
Chang Hyuck Choi, Minho Kim, Han Chang Kwon, Sung June Cho, Seongho Yun, Hee-Tak Kim, Karl J.J. Mayrhofer, Hyungjun Kim, Minkee Choi
Nature Communications, Vol. 7, page 10922 (March 8, 2016)
[Abstract]
Maximum atom efficiency as well as distinct chemoselectivity is expected for electrocatalysis on atomically dispersed (or single-site) metal centers, but its realization remains challenging so far because carbon as the most widely used electrocatalyst support cannot effectively stabilize them. Here we report that a sulfur-doped zeolite-templated carbon, simultaneously exhibiting extra-large sulfur content (17 wt% S) as well as a unique carbon structure (i.e., highly curved 3-dimensional networks of graphene nanoribbons), can stabilize a relatively high loading of Pt (5 wt%) in the form of atomically dispersed platinum species. In the oxygen reduction reaction (ORR), this catalyst does not follow a conventional 4-electron pathway producing H2O, but selectively produces H2O2 even over extended times without significant degradation of the activity. Thus this approach constitutes a potentially promising route for producing important fine chemical H2O2, and also offers new opportunities for tuning selectivity of other electrochemical reactions on various metal catalysts.
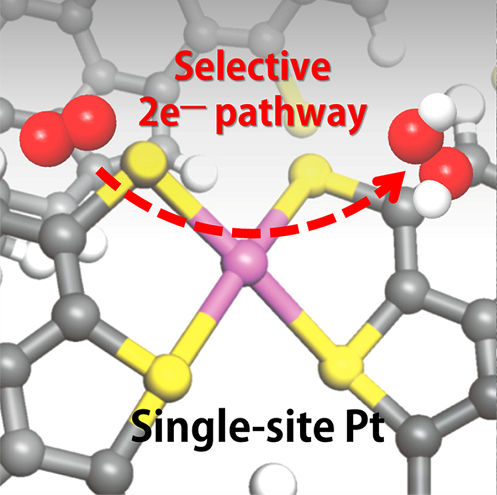
그림1. 백금 단일원자 촉매에서의 과산화수소(H2O2) 생성반응 모식도
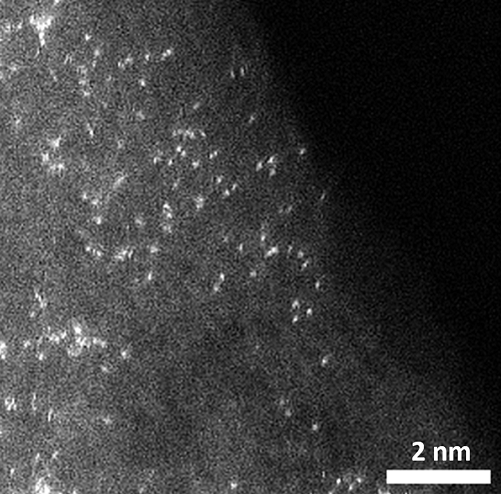
그림 2. HAADF-STEM 분석법을 통해 관찰한 백금 단일 원자 사진(탄소: gray, 수소: white, 황 : yellow, 백금 : purple)
���������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������



