A research team led by Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering at KAIST has developed water-based zinc/bromine redox flow batteries (ZBBs) with the best life expectancy by identifying and solving the deterioration issue with zinc electrodes.
Professor Kim said, "We presented a new technology to overcome the life limit of water-cell batteries. It is even cheaper than conventional lithium-ion batteries, and it is expected that it can also contribute to the increase of renewable energy use and the safe supply of energy storage systems with high efficiency."
ZBBs were found to have stable life spans with more than 5,000 cycles, even at a high current density of 100 mA/cm2. It was also confirmed that it represents the highest output and life expectancy compared to conventional redox flow batteries (RFBs) reported, which use other redox couples such as zinc-bromine, zinc-iodine, zinc-iron, and vanadium.
With increasing demands for energy, various technologies asssociated with energy storage system (ESS) are getting more important, which improve the efficiency of energy utilization by storing new and late-night power in large quantities and supplying it to the grid if necessary to supplement the intermittent nature of renewable energy and meet peak power demand. However, lithium-ion batteries (LIBs), which are currently the core technology of ESSs, have brought serious concerns about their inherent risk of ignition and fire.
As a result, researchers have focused on the development of water-based RFBs, and particularly ZBBs possess their own merits in use of ultra-low-cost bromide (ZnBr2) as an active material, along with the feature of high cell voltage and high energy density. However, they have not been commercialized due to the short life span, mainly related to the electrodes in which the dendrite growth of zinc metals during operation leads to internal short circuits.
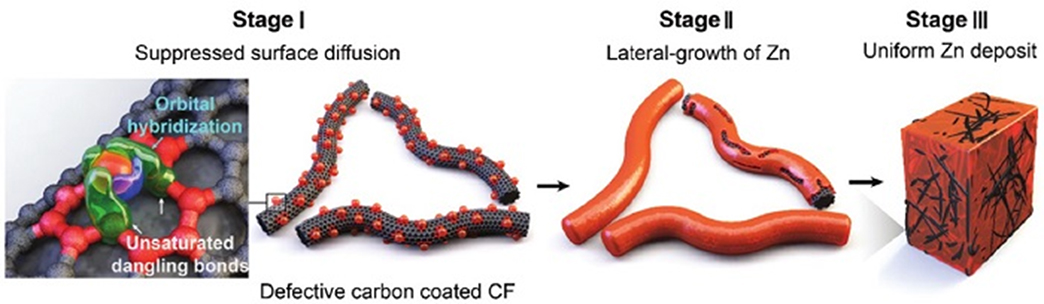
The research team noted that a self-aggregation of zinc occurs through the surface diffusion on the carbon electrode surface with low surface energy, and they found out that a defective carbon structure prevents such surface diffusion of zinc so that dendrites were not produced. The strong bonding between zinc adatoms and single vacancy defects inhibits the surface diffusion at the initial deposition, enabling uniform zinc nuclear production/growth. As a result, the research teamwas able to achieve the cycling stability, corresponding to more than 5,000 cycles at a high charge current density (100 mA/cm2).
It is expected that this technology can drive safe and cheap redox flow batteries for long life, boosting the development of ESS system. This research was published on Energy and Environmental Science (title: Dendrite-free Zn electrodeposition triggered by interatomic orbital hybridization of Zn and single vacancy carbon defects for aqueous Zn-based flow batteries), also featured as a journal cover.





